
Effector Therapeutics Inc
$ 0.00
0.00%
24 Feb - close price
- Market Cap 940 USD
- Current Price $ 0.00
- High / Low $ 0.00 / 0.00
- Stock P/E N/A
- Book Value 0.21
- EPS -12.57
- Next Earning Report -
- Dividend Per Share N/A
- Dividend Yield 0 %
- Next Dividend Date -
- ROA -0.83 %
- ROE -9.47 %
- 52 Week High 0.00
- 52 Week Low 0.00
About
Effector Therapeutics Inc (EFTR) is a clinical-stage biopharmaceutical company at the forefront of targeted cancer therapies, leveraging selective modulation of RNA translation to develop innovative small molecule treatments. By focusing on altering protein synthesis specifically in tumor cells, Effector Therapeutics addresses significant unmet medical needs within oncology. With a diverse pipeline of product candidates and a strong commitment to precision medicine, the company aims to enhance treatment outcomes and patient quality of life in a dynamic biotechnology market.
Analyst Target Price
$10.00
Quarterly Earnings
| Mar 2024 | Dec 2023 | Sep 2023 | Jun 2023 | Mar 2023 | Dec 2022 | Sep 2022 | Jun 2022 | Mar 2022 | Dec 2021 | Sep 2021 | Jun 2021 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reported Date | 2024-05-09 | 2024-03-25 | 2023-11-13 | 2023-08-08 | 2023-05-09 | 2023-03-08 | 2022-11-07 | 2022-08-09 | 2022-05-10 | 2022-03-16 | 2021-11-08 | 2021-08-31 |
| Reported EPS | -2.16 | -3.42 | -0.13 | -0.17 | -0.24 | -0.22 | -0.23 | -0.17 | 0.07 | 0.44 | 0.42 | -0.37 |
| Estimated EPS | -2.16 | -1.65 | -0.12 | -0.2 | -0.14 | -0.24 | -0.21 | -0.16 | -0.24 | -0.24 | -0.2 | -0.285 |
| Surprise | 0 | -1.77 | -0.01 | 0.03 | -0.1 | 0.02 | -0.02 | -0.01 | 0.31 | 0.68 | 0.62 | -0.085 |
| Surprise Percentage | 0% | -107.2727% | -8.3333% | 15% | -71.4286% | 8.3333% | -9.5238% | -6.25% | 129.1667% | 283.3333% | 310% | -29.8246% |
Next Quarterly Earnings
| Reported Date |
| Fiscal Date Ending |
| Estimated EPS |
| Currency |
Next Dividend Records
| Dividend per share (year): | - |
| Dividend Yield | - |
| Next Dividend Date | - |
| Ex-Dividend Date | - |
Recent News: EFTR
2024-06-24 07:21:00
eFFECTOR Therapeutics (EFTR) has decided to terminate its employees and wind down all operations, including exploring strategic alternatives for its development programs. The company also plans to voluntarily request the delisting of its securities. Craig Jalbert has been appointed CEO, President, Treasurer, Secretary, and sole board member, as shares dropped 65% in pre-market trading.
2024-06-24 00:09:16
eFFECTOR Therapeutics, Inc. announced it has terminated employees and will wind down operations, including seeking strategic alternatives for its development programs. The decision was made by the board of directors, which also appointed Craig R. Jalbert as CEO, President, Treasurer, Secretary, and sole board member. The company plans to voluntarily request a delisting of its securities from Nasdaq, as it no longer meets continued listing requirements.

2024-05-20 20:36:59
eFFECTOR Therapeutics announced a collaboration with the Dana-Farber Cancer Institute for an investigator-sponsored Phase 2 clinical trial. The trial will evaluate zotatifin in combination with abemaciclib and letrozole for treating ER+ endometrial cancer and low-grade serous ovarian cancer. This initiative aims to address treatment resistance and improve outcomes in these cancers by targeting the eIF4A pathway.
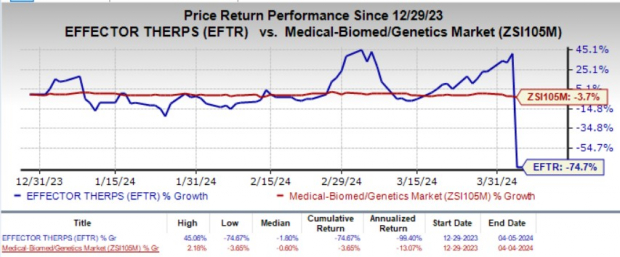
2024-04-05 01:57:25
eFFECTOR Therapeutics (EFTR) shares dropped 82% after its mid-stage NSCLC study for tomivosertib failed to meet its primary endpoint, leading to the discontinuation of the development program for that indication. The company will now focus resources on its zotatifin candidate for breast cancer, with a registrational study planned for later in 2024. A separate investigator-sponsored study for tomivosertib in acute myeloid leukemia will continue, as its rationale is distinct from the failed NSCLC study.
2024-04-05 01:09:49
eFFECTOR Therapeutics' stock (EFTR) plummeted 82% after its mid-stage non-small cell lung cancer (NSCLC) study for tomivosertib failed to meet its primary endpoint. The company has consequently abandoned the tomivosertib program for frontline NSCLC and will redirect its focus to zotatifin, another clinical-stage candidate for estrogen receptor-positive breast cancer. A separate study for tomivosertib in acute myeloid leukemia (AML) will continue, as its rationale is distinct from the NSCLC study.

2024-04-04 08:30:00
eFFECTOR Therapeutics announced topline results from its Phase 2 KICKSTART trial for tomivosertib combined with pembrolizumab in non-small cell lung cancer, which did not meet its primary endpoint for progression-free survival. While tomivosertib showed modest activity, the company will not pursue further development in frontline NSCLC and plans to focus on advancing zotatifin for ER+ breast cancer. An investigator-sponsored trial for tomivosertib in acute myeloid leukemia will continue.


