
Beam Therapeutics Inc
$ 32.29
13.98%
24 Feb - close price
- Market Cap 3,276,626,000 USD
- Current Price $ 32.29
- High / Low $ 32.83 / 29.51
- Stock P/E N/A
- Book Value 9.80
- EPS -4.41
- Next Earning Report 2026-03-03
- Dividend Per Share N/A
- Dividend Yield 0 %
- Next Dividend Date -
- ROA -0.24 %
- ROE -0.47 %
- 52 Week High 36.44
- 52 Week Low 13.53
About
Beam Therapeutics Inc., a biotechnology company, develops precision genetic drugs for patients with serious illnesses in the United States. The company is headquartered in Cambridge, Massachusetts.
Analyst Target Price
$49.53
Quarterly Earnings
| Sep 2025 | Jun 2025 | Mar 2025 | Dec 2024 | Sep 2024 | Jun 2024 | Mar 2024 | Dec 2023 | Sep 2023 | Jun 2023 | Mar 2023 | Dec 2022 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reported Date | 2025-11-04 | 2025-08-05 | 2025-05-05 | 2025-02-25 | 2024-11-05 | 2024-08-06 | 2024-05-07 | 2024-02-27 | 2023-11-08 | 2023-08-08 | 2023-05-10 | 2023-02-28 |
| Reported EPS | -1.1 | -1 | -1.2421 | -1.09 | -1.17 | -1.11 | -1.21 | 1.73 | -1.22 | -1.08 | -1.33 | -0.54 |
| Estimated EPS | -1.05 | -1.11 | -1.1714 | -1.2158 | -1.16 | -1.13 | -1.42 | -1.01 | -1.36 | -1.42 | -1.34 | -1.33 |
| Surprise | -0.05 | 0.11 | -0.0707 | 0.1258 | -0.01 | 0.02 | 0.21 | 2.74 | 0.14 | 0.34 | 0.01 | 0.79 |
| Surprise Percentage | -4.7619% | 9.9099% | -6.0355% | 10.3471% | -0.8621% | 1.7699% | 14.7887% | 271.2871% | 10.2941% | 23.9437% | 0.7463% | 59.3985% |
Next Quarterly Earnings
| Dec 2025 | |
|---|---|
| Reported Date | 2026-03-03 |
| Fiscal Date Ending | 2025-12-31 |
| Estimated EPS | -1.01 |
| Currency | USD |
Next Dividend Records
| Dividend per share (year): | - |
| Dividend Yield | - |
| Next Dividend Date | - |
| Ex-Dividend Date | - |
Recent News: BEAM
2026-02-25 07:51:52
Beam Therapeutics has secured a $500 million strategic financing facility with Sixth Street, with $100 million funded at closing and an additional $400 million tied to milestones. This seven-year, non-dilutive capital aims to support the potential commercial launch of ristoglogene autogetemcel (risto-cel) for sickle cell disease. The financing is expected to bolster Beam's balance sheet, manage commercialization costs for risto-cel, and allow the company to further invest in its pipeline and precision genetic medicines vision.

2026-02-24 19:51:52
Beam Therapeutics has secured a non-dilutive financing agreement with Sixth Street for up to $500 million to fund the potential launch and commercialization of its sickle cell gene therapy, risto cel. This debt financing, with a 7-year term and approximately 10% interest, avoids immediate equity dilution for shareholders. The capital will support the company's efforts without issuing new shares, influencing its spending on launch preparation and market access if the therapy progresses.
2026-02-24 11:51:52
Beam Therapeutics Inc. has released its annual 10-K report, highlighting significant financial and operational achievements, including increased license revenue and a reduced net loss, primarily due to a collaboration with Pfizer. The company, which specializes in base editing technology, is advancing lead programs for sickle cell disease and alpha-1 antitrypsin deficiency, with risto-cel in Phase 1/2 clinical trials and a BLA submission planned by year-end 2026. Despite financial and operational risks, Beam Therapeutics aims to expand its platform and secure additional capital to support its growth strategy and clinical advancements.

2026-02-23 21:27:21
Beam Therapeutics Inc. (Nasdaq: BEAM) announced it will host an investor webcast on February 24, 2026, at 8:00 a.m. ET to discuss its fourth quarter and full-year 2025 financial results. The biotechnology company will also introduce a new liver-targeted genetic disease program during the webcast. Beam Therapeutics specializes in developing precision genetic medicines using base editing technology.

2026-02-23 21:05:16
Beam Therapeutics announced it will host an investor webcast on Tuesday, February 24, 2026, at 8:00 a.m. ET. The webcast will cover the company's fourth-quarter and full-year 2025 financial results and introduce a new liver-targeted genetic disease program. Beam Therapeutics specializes in precision genetic medicines using base editing technology.
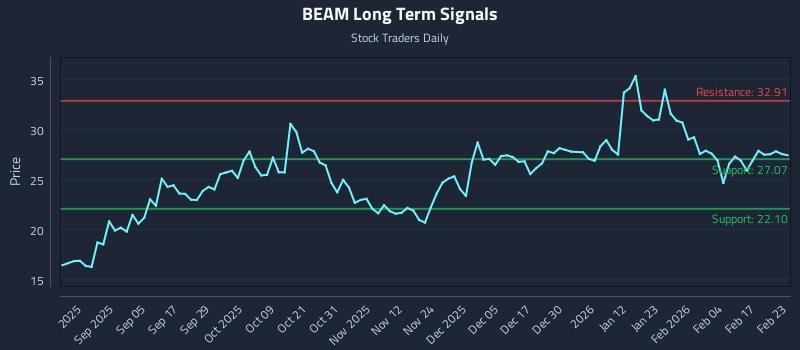
2026-02-23 10:46:16
This article analyzes Beam Therapeutics Inc. (NASDAQ: BEAM), noting a positive near-term sentiment within a long-term strength context despite mid-term weakness. It highlights an exceptional risk-reward setup, with specific support and resistance levels, and provides three distinct AI-generated trading strategies for different risk profiles.


